Protease Inhibitor & Reduction Agents
AEBSF / 6-Aminocaproic acid / APMSF / Aprotinin /
Benzamidine / Bestatin / Chymostatin
/ E-64 / EDTA / EGTA / Leupeptin /
Pepstatin A / PMSF
TCEP / DTE / DTT / 2-Mercaptoethanol .....
|
A protease (also called a peptidase or proteinase) is an enzyme that performs proteolysis: protein
catabolism by hydrolysis of peptide
bonds.
Proteases have evolved
multiple times, and different classes of protease can perform the same reaction by
completely different catalytic
mechanisms. Proteases can be found in Animalia, Plantae, Fungi, Bacteria, Archaea and viruses.
By splitting the peptide
bonds that link amino acid residues, the proteases
are involved in digesting long protein chains into shorter fragments. Some detach the terminal amino
acids from the protein chain (exopeptidases, such as aminopeptidases, carboxypeptidase
A); others attack internal peptide bonds of a protein (endopeptidases, such as trypsin, chymotrypsin, pepsin, papain, elastase).
Proteases are also secreted to process food, e.g. in the intestinal tract of
animals.
https://en.wikipedia.org/wiki/Protease#Enzymatic_function_and_mechanism.
Protease inhibitor
|
|
In biology and biochemistry, protease inhibitors are molecules that inhibit the function of proteases (enzymes that aid the breakdown of proteins). Many naturally occurring
protease inhibitors are proteins.
In medicine, protease inhibitor is
often used interchangeably with alpha 1-antitrypsin (A1AT,
which is abbreviated PI for this reason).[1] A1AT is indeed
the protease inhibitor most often involved in disease, namely in alpha-1
antitrypsin deficiency.
https://en.wikipedia.org/wiki/Protease_inhibitor_(biology)
Protease Inhbitor s are abundant in every organism inside
their cells and here fulfill a crucial role in regulating the life cycle of
proteins, activating pro-enzymes or eliminating problematic proteins.
|
|
Protease Inhibitors in protein purification procedures |

|
|
Whenever proteins are analyzed in biological samples or purified from a natural
source, protease activity is a potential threat.
During sample preparation, cells are frequently lysed and in this way they set
free high amounts of protease activities that may digest the proteins of
interest.
The days of work for cell culture and protein sample preparation can be
destroyed within a few seconds.
Labs generally apply two basic strategies to knock out such unwanted proteolytic
activities:
(a) Cooling the sample or cell lysate, and
(b) Adding chemical inhibitors of proteases.
The most common proteases are the serine proteases Chymotrypsin, Kallikrein,
Plasmin, Proteinase K, Thrombin and Trypsin. Hence their deactivation after
cellular homogenization is very important in the isolation of proteins in order
to ensure satisfactory protein purification yields.
Our team partner, the Biosynth offers
most of the successfully used protease inhibitors that offer both high purity
and the requisite grade for use in biochemistry laboratories and production
scale purification process:
|
|
|
4-(2-Aminoethyl)-benzenesulfonylfluoride hydrochloride (AEBSF)
https://en.wikipedia.org/wiki/AEBSF
Target Enzymes:
Serine Proteases |

|
AEBSF or 4-(2-aminoethyl)benzenesulfonyl
fluoride hydrochloride is a water-soluble, irreversible serine
protease inhibitor with a molecular weight of 239.5 Da. It inhibits proteases like chymotrypsin, kallikrein, plasmin, thrombin, and trypsin. The specificity is similar to the
inhibitor PMSF, nevertheless AEBSF is more stable at low pH values. Typical usage is 0.1 -
1.0 mM.
Mechanism of action:
Both AEBSF and PMSF are sulfonyl
fluorides and are sulfonylating agents.[1]Sulfonyl fluorides act by reacting
with the hydroxy
group of the active
site serine residue to form a sulfonyl enzyme derivative. This derivative may be stable for long
periods of time except at high pH.[2]AEBSF are irreversible serine protease inhibitors and are, thus, part of
most homogenization buffers and added to cell lysates.
Deactivation (irreversible inhibition) occurs through esterification of the serine
hydroxyl group.
While PMSF not only deactivates serine proteases but also any other enzyme that
contains serine in its active site, and therefore cannot be used if the
biological activity of such an enzyme needs to be maintained, the AEBSF is often
preferred over PMSF or DFP (diisopropylfluorophosphate), because it is
less toxic and water soluble.
Aqueous AEBSF solutions are stable at slightly acidic pH values.
|
|
|
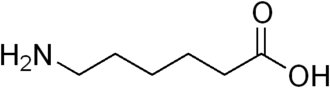
6-Aminocaproic acid
https://en.wikipedia.org/wiki/Aminocaproic_acid
Target
Enzymes:
Serine Proteases |
|
|
Aminocaproic acid (also
known as ε-aminocaproic
acid, ε-Ahx,
or 6-aminohexanoic
acid) is a derivative and analogue of the amino acid lysine, which makes it an effective inhibitor for enzymesthat bind that particular residue. Such enzymes
include proteolytic enzymes
like plasmin, the enzyme responsible for fibrinolysis. For this reason it is effective in
treatment of certain bleeding disorders, and it is marketed as Amicar.
Aminocaproic acid is also an intermediate in the polymerization of Nylon-6,
where it is formed by ring-opening hydrolysis of caprolactam.
|
|
|
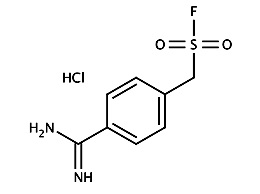
4-Amidinophenylmethansulfonylfluorid - Hydrochlorid (APMSF)
https://www.biosynth.com/products/products/A-4895.html
https://en.wikipedia.org/wiki/PMSF
Target Enzymes:
Serine Proteases,
Trypsin,
Thrombin,
Factor Xa,
Plasmin. |
|
Irreversiblely inhibit serine
proteases specifically for lysine
or arginine substrate.
Also new discovered proteases characterizations.
|
|
|
Aprotinin
https://en.wikipedia.org/wiki/Aprotinin#Chemistry
Target Enzymes:
Plasmin,
Kallikrein,
Trypsin,
Chymotrypsin |

|
The drug aprotinin (Trasylol, previously Bayer and now Nordic Group pharmaceuticals), is a small protein bovine pancreatic trypsin inhibitor (BPTI), or basic trypsin inhibitor
of bovine pancreas, which is an antifibrinolytic molecule that inhibits trypsin and related proteolytic enzymes.
In vitro use : Small amounts of aprotinin can be added to tubes of drawn blood
to enable laboratory measurement of certain rapidly degraded proteins such as glucagon.
In cell biology aprotinin is used as an enzyme
inhibitor to prevent protein degradation during lysis or homogenization of cells and tissues.
Aprotinin can be labelled with fluorescein isothiocyanate. The conjugate retains
its antiproteolytic and carbohydrate-binding properties[21]and
has been used as a fluorescent histochemical reagent for staining
glycoconjugates (mucosubstances) that are rich in uronic or sialic acids.[22]
BPTI is one of the most thoroughly studied proteins in terms of structural biology, experimental and computational dynamics,
mutagenesis, and folding
pathway. It was one of the earliest protein crystal structures
solved, in 1970 in the laboratory of Robert Huber,[28] and
was the first protein to have its structure determined
by NMR spectroscopy, in the laboratory
of Kurt Wuthrich at the ETH in Zurich in the early 1980s.[29][30]
Aprotinin from bovine lung : Aprotinin
is a small protein but a powerful inhibitor that prevents activity of several
serine proteases (trypsin, chymotrypsin, plasmin, and kallikrein) already at low
inhibitor concentrations.
|
|
|
Benzamidine hydrochloride monohydrate (Benzamidine)
https://en.wikipedia.org/wiki/Benzamidine
Target Enzymes:
Serine Proteases |

|
Benzamidine is a reversible competitive inhibitor of trypsin, trypsin-like enzymes and serine proteases.It
is often used as a ligand in protein crystallography to prevent proteases from degrading a protein of interest; the triangular
diamine group at the bottom gives it a very obvious 'stick-man' shape which
shows up in difference density maps. The benzamidine moiety is also found in some
pharmaceuticals, like dabigatran.
Benzamidine is a competitive inhibitor of serine proteases.
Benzamidine is frequently added to cell lysates, especially yeast cell extracts.
It is also a preferred protease inhibitor in protein crystallography.
p-Aminobenzamidine immobilized on agarose is used in the isolation of serine
proteases by affinity chromatography. |
|
|
Bestatin
https://en.wikipedia.org/wiki/Ubenimex
Target Enzymes:
Aminopeptidases
|

|
Ubenimex (INN), also known more commonly as bestatin, is a competitive, reversible protease
inhibitor. It is an inhibitor of arginyl
aminopeptidase (aminopeptidase B),[2] leukotriene
A4 hydrolase(a zinc
metalloprotease that displays both epoxide
hydrolase and aminopeptidase activities),[3] alanyl
aminopeptidase (aminopeptidase M/N),[4] leucyl/cystinyl
aminopeptidase(oxytocinase/vasopressinase),[5][6] and membrane
dipeptidase (leukotriene D4 hydrolase).
It is being studied for use in the treatment of acute
myelocytic leukemia[7] and lymphedema[8]. It is derived from Streptomyces olivoreticuli.[9] Ubenimex has been found to inhibit
the enzymatic degradation of oxytocin, vasopressin, enkephalins, and various other peptides and compounds.[citation
needed] |
|
|
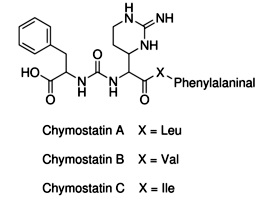
Chymostatin
https://www.biosynth.com/products/products/C-5981.html
Target Enzymes:
Serine Proteases,
Cysteine Proteases,
Papain. |
|
Strongly inhibit proteases such as
chymotrypsin and
analogous serine proteinases,
papain, chymases, and
cathepsins A,B,C, B, H,
and L etc that belong to lysosomal cysteine proteinases catagory and the soluble
Ca2+ activated proteinase.
Compositions of Chymostatin with other protease inhibitors is often applied for
use with plant extracts, especially for young plant tissues which may frequently
express serine protease with sensitivity to chomostatin.
Chomostatin has weak inhibitions for human
leucocyte elastase.
|
|
|
E-64
https://www.biosynth.com/products/products/E-0010.html
Target Enzymes:
Cysteine Proteases
(Papain,
Cathepsin) |

|
E-64 irreversibly inhibit cysteine proteases (calpain, papain, and
cathepsin B, cathepsin L) with high selectivity and potency, acting by forming a
thioether bond with the thiol functional group of the active cysteine, and does
not affect the functional thiol group of cysteine residues in non-protease
enzymes, as creatine kinase or L-lactate dehydrogenase.
Trypsin is the only one serine proteases that E-64 inhibits.
E-64 have capability to restore HIV defective immune responses and inhibition of
activation-induced programmed cell death.
E-64 has application for cysteine proteases affinity purification, where it is
coupled to a thiolated affinity matrix as an effective ligand.
The coupled E-64 no longer binds substrates irreversibly yet with specificity
retained.
|
|
|
EDTA
https://en.wikipedia.org/wiki/EDTA
Target Enzymes:
Metalloproteases |

|
|
|
|
|
EGTA
https://en.wikipedia.org/wiki/EGTA_(chemical)
Target Enzymes:
Ca(II)-Proteases |

|
EGTA (ethylene glycol-bis(β-aminoethyl
ether)-N,N,N',N'-tetraacetic acid), also known as egtazic acid (INN, USAN),[1] is an aminopolycarboxylic
acid, a chelating
agent. It is a colourless solid that is
related to the better known EDTA.
Compared to EDTA, it has a lower affinity for magnesium, making it more selective for calciumions. It is useful in buffer
solutions that resemble the environment in
living cells[2] where calcium ions are usually at
least a thousandfold less concentrated than magnesium.
EGTA has also been used experimentally for the treatment of animals with cerium
poisoning and for the separation of thorium from the mineral monazite.
EGTA is used as a compound in elution buffer in the Protein Purification technique known
as
Tandem
Affinity Purification,
in which recombinant fusion proteins are bound to calmodulin beads and eluted
out by adding EGTA.
EGTA is often employed in dentistry and endodontics for the removal of the smear
layer.
|
|
|
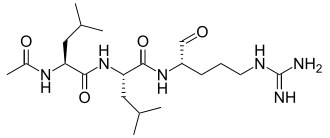
Leupeptin
https://en.wikipedia.org/wiki/Leupeptin
Target Enzymes:
Serine and
Cysteine Proteases |
|
.Leupeptin, also known as N-acetyl-L-leucyl-L-leucyl-L-argininal, is a naturally occurring protease inhibitor that can inhibit
cysteine, serine and threonine peptidases.
It is often used during in vitro experiments when a specific enzymatic reaction is being
studied. When cells are lysed for these studies, proteases, many of which are contained within lysosomes, are released.
These proteases, if freely present in the lysate, would destroy any products
from the reaction being studied, and make the experiment uninterpretable.
For example, leupeptin could be used in a calpain extraction to keep calpain from being hydrolyzed by
specific proteases. The suggested concentration is 1-10 µM (0.5-5 µg/ml).
Leupeptin is an organic compound produced by actinomycetes, which inhibits serine, cysteine and threonine proteases.
Leupeptin inhibits serine proteinases (trypsin (Ki=3.5 nM), plasmin (Ki= 3.4 nM), porcine kallikrein), and cysteine proteinases (papain, cathepsin B (Ki = 4.1 nM), endoproteinase Lys-C).
It does not inhibit α-chymotrypsin or thrombin.
Leupeptin is a competitive transition state inhibitor and its inhibition may be
relieved by an excess of substrate.
Leupeptin is soluble in water (stable for 1 week at 4 °C and 1 month at −20 °C),
ethanol, acetic acid and DMF.
It can be given topically for middle and inner ear infections.[1] |
|
|
Pepstatin A
https://en.wikipedia.org/wiki/Pepstatin
https://en.wikipedia.org/wiki/Statine
Target Enzymes:
Aspartic Proteases (eg. HIV) |

|
Pepstatin is a potent inhibitor of aspartyl
proteases.
It is a hexa-peptide containing the unusual amino acid statine (Sta, (3S,4S)-4-amino-3-hydroxy-6-methylheptanoic acid), having the sequence
Isovaleryl-Val-Val-Sta-Ala-Sta (Iva-Val-Val-Sta-Ala-Sta).[1] It was originally isolated from
cultures of various species of Actinomyces[1] due to its ability to inhibit pepsin at picomolar concentrations.[2]
Pepstatin A is well known to be an
inhibitor of aspartic proteinases such as pepsin, cathepsins D and E. |
|
|
Phenylmethylsulfonyl fluoride (PMSF)
https://en.wikipedia.org/wiki/PMSF
Target Enzymes:
Serine Proteases |

|
.In biochemistry, phenylmethane sulfonyl fluoride or phenylmethylsulfonyl fluoride (PMSF) is a serine
protease inhibitorcommonly used in the preparation of cell
lysates. PMSF does not inhibit all serine proteases[citation
needed]. It is rapidly degraded in water and stock solutions are usually made up in
anhydrous ethanol, isopropanol, corn
oil, or DMSO.
PMSF binds specifically to the active site serine residue in a serine protease. It does not bind to any other serine residues
in the protein.
The median lethal dose is less than 500 mg/kg[citation needed].
PMSF is a cytotoxic chemical
which should be handled only inside a fume hood.
PMSF is commonly used in protein solublization in order to deactivate proteases
from digesting proteins of interest after cell lysis. |
|
|
|
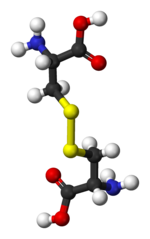
Reduction Agents in Downstream processing |
|

|

|

|
|
|
The disulfide bonds are extremely rare in cytosolic
proteins, since the cytosol (intracellular fluid) is generally a reducing
environment.
https://en.wikipedia.org/wiki/Protein_structure#Tertiary_structure
https://en.wikipedia.org/wiki/Disulfide#Occurrence_in_proteins
Disulfide Reduction Reagents reduce oxidation of a protein sample, inhibit the
oxidation of free sulfhydryl residues, maintaining SH groups in the reduced
state, and its ability thereby, preserve enzymatic activity.
Its usefulness also stems from its water solubility, reduced odor, and lower
toxicity. |
|
|
Tris-(2-carboxyethyl)phosphine HCl (TCEP)
https://en.wikipedia.org/wiki/TCEP |

|
TCEP (tris(2-carboxyethyl)phosphine) is a reducing agent frequently used in
biochemistry and molecular biology applications.[1]
It is often prepared and used as a hydrochloride salt (TCEP-HCl) with a
molecular weight of 286.65 gram/mol.
It is soluble in water and available as a stabilized solution at neutral pH and
immobilized onto an agarose support to facilitate removal of the reducing agent.
TCEP is often used as a reducing agent to break disulfide bonds within and
between proteins as a preparatory step for gel electrophoresis.
Compared to the other two most common agents used for this purpose (dithiothreitol and
β-mercaptoethanol), TCEP has the advantages of being odorless, a more powerful
reducing agent, an irreversible reducing agent (in the sense that TCEP does not
regenerate—the end product of TCEP-mediated disulfide cleavage is in fact two
free thiols/cysteines), more hydrophilic, and more resistant to oxidation in
air.[2]
It also does not reduce metals used in immobilized metal affinity
chromatography.
TCEP is particularly useful when labeling cysteine residues with maleimides.
TCEP can keep the cysteines from forming di-sulfide bonds and unlike
dithiothreitol and β-mercaptoethanol, it will not react as readily with the
maleimide.[2] However, TCEP has been reported to react with maleimide under
certain conditions.[3][4]
TCEP is also used in the tissue homogenization process for RNA isolation.[5]
. |
|
|
1,4-Dithioerythritol (DTE)
https://en.wikipedia.org/wiki/Dithioerythritol
|

|
Dithioerythritol (DTE) is
a sulfur containing sugar derived from the corresponding 4-carbon monosaccharide erythrose.
It is an epimer of dithiothreitol (DTT).
The molecular formula for DTE is C4H10O2S2.
Like DTT, DTE makes an excellent reducing agent, although its standard reduction potential is not quite as negative, i.e., DTE is
slightly less effective at reducing than DTT. This is presumably because the
orientation of the OH groups in its cyclic disulfide-bonded form
(oxidized form) is less stable due to greater steric
repulsion than their orientation in the disulfide-bonded form of DTT.
In the disulfide-bonded form of DTT, these hydroxyl groups are trans to each other, whereas they are cis to each
other in DTE. |
|
|
1,4-Dithiothreitol (Cleland's Reagent, DTT)
https://en.wikipedia.org/wiki/Dithiothreitol
|
|
Dithiothreitol (DTT) is the common name for a small-molecule redox reagent also known as Cleland's reagent.[2]
DTT has an epimeric('sister') compound, dithioerythritol (DTE).
DTT is used as a reducing or "deprotecting" agent for thiolated DNA.
The terminal sulfur atoms of thiolated DNA have a tendency to form dimers in solution, especially in the presence of oxygen. Dimerization
greatly lowers the efficiency of
subsequent coupling reactions such as DNA immobilization on gold in biosensors.
The DTT removal procedure is often called "desalting."
Generally, DTT is used as a protecting agent that prevents oxidation of thiol
groups.
DTT is frequently used to reduce the disulfide
bonds of proteins and, more generally, to prevent intramolecular and intermolecular disulfide bonds from forming
between cysteine residues of proteins.
However, even DTT cannot reduce buried (solvent-inaccessible) disulfide bonds,
so reduction of disulfide bonds is sometimes carried out under denaturing
conditions (e.g., at high temperatures, or in the presence of a strong denaturant such as 6 M guanidinium
chloride, 8 M urea, or 1% sodium
dodecylsulfate). |
|
|
2-Mercaptoethanol
https://en.wikipedia.org/wiki/2-Mercaptoethanol |

|
2-Mercaptoethanol (also β-mercaptoethanol, BME, 2BME, 2-ME or β-met) is the chemical
compound with the formula HOCH2CH2SH. ME or βME, as it is commonly abbreviated, is used to reduce disulfide
bonds and can act as a biological antioxidant by scavenging hydroxyl radicals (amongst others). It is widely used because
the hydroxyl group confers solubility in water and lowers the volatility. Due to
its diminished vapor pressure, its odor, while unpleasant, is less objectionable
than related thiols.
Applications:
Reducing
proteins,
Preventing protein oxidation,
Denaturing
ribonucleases.
|
|
|
|