|
Macro Ion Exchange and Adsorbent Resins
Softening / Demineralisation / Dealkalisation / Nuclear Grade Food Grade /
Polymeric Adsorbent / Polymeric Catalyst
https://en.wikipedia.org/wiki/Ion-exchange_resin
https://en.wikipedia.org/wiki/Adsorption

Ion-exchange resin beads |
An ion-exchange
resin or ion-exchange
polymer is
a resin or polymer
that acts as a medium for ion exchange.
It is an insoluble matrix
(or support structure) normally in the form of small (0.25–0.5 mm radius) microbeads,
usually white or yellowish, fabricated from an organicpolymer substrate.
The beads are typically porous,
providing a large surface area on
and inside them. The trapping of ions occurs
along with the accompanying release of other ions, and thus the process is
called ion exchange. There are multiple types of ion-exchange resin. Most
commercial resins are made of polystyrene sulfonate.[1] |
|
Ion-exchange resins are widely used in different separation,
purification, and decontamination processes. The most common examples are water softening and water purification.
In many cases ion-exchange resins were introduced in such processes as a more
flexible alternative to the use of natural or artificial zeolites.
Also, ion-exchange resins are highly effective in the biodiesel filtration
process. |
|
Most typical ion-exchange resins are based on crosslinked polystyrene. The actual ion-exchanging sites are
introduced after polymerisation. Additionally, in the case of polystyrene,
crosslinking is introduced by copolymerisation of styrene and a few percent of divinylbenzene. Crosslinking decreases
ion-exchange capacity of the resin and prolongs the time needed to accomplish
the ion-exchange processes but improves the robustness of the resin. Particle
size also influences the resin parameters; smaller particles have larger outer
surface, but cause larger head loss in the column processes.[2]
Besides being made as bead-shaped materials, ion-exchange resins are also
produced as membranes. These ion-exchange membranes, which are made of
highly cross-linked ion-exchange resins that allow passage of ions, but not of
water, are used for electrodialysis.
Four main types of ion-exchange resins differ in their functional groups:
- strongly acidic, typically featuring sulfonic acid groups, e.g. sodium polystyrene sulfonate or polyAMPS,
- strongly basic, typically featuring quaternary amino groups, for example, trimethylammonium groups, e.g. polyAPTAC),
- weakly acidic, typically featuring carboxylic acid groups,
- weakly basic, typically featuring primary,
secondary, and/or tertiary amino groups, e.g. polyethylene amine.
Specialised ion-exchange resins are also known such as chelating resins (iminodiacetic acid, thiourea-based resins, and many others). Anion resins
and cation resins are the two most common resins used in the ion-exchange
process. While anion resins attract negatively charged ions, cation resins
attract positively charged ions.....
|
|
The presence of certain metal ions like calcium and magnesium principally as bicarbonates, chlorides, and sulfatesin water causes a variety of problems.[1]
Hard water leads to the buildup of limescale, which can foul plumbing, and promote galvanic corrosion.[2] In industrial
scale water softening plants, the effluent flow from the re-generation process
can precipitate scale that can interfere with sewage systems.[3]
The slippery feeling experienced when using soap with
soft water occurs because soaps tend to bind to fats in the surface layers of skin, making soap
molecules difficult to remove by simple dilution. In contrast, in hard-water
areas, the rinse water contains calcium or magnesium ions that form insoluble salts, effectively removing the residual soap from the
skin but potentially leaving a coating of insoluble stearates on tub and shower
surfaces, commonly called soap scum.[4]
|

Limescale in
a PVC pipe |
The desirability of these competing effects varies by
personal preference, and those who dislike the effects of soft water may choose
to harden the water by adding chemicals such as baking soda, calcium chloride, or magnesium sulfate.[5]
https://en.wikipedia.org/wiki/Water_softening#Rationale |
Water softening is
the removal of calcium, magnesium,
and certain other metal cations in hard water.
The resulting soft water requires less soapfor
the same cleaning effort, as soap is not wasted mopping up calcium ions. Soft
water also extends the lifetime of plumbing by
reducing or eliminating scale build-up
in pipes and fittings. Water softening is usually achieved using lime softening or ion-exchange resins but
is increasingly being accomplished using nanofiltration or reverse osmosis membranes.
|

Control room and schematics of the water purification plant of Lac de Bret,
Switzerland
|
Water purification is the process of
removing undesirable chemicals, biological contaminants, suspended solids, and
gases from water. The goal is to produce water fit for specific purposes. Most
water is purified and disinfected for human consumption (drinking water), but water purification may also
be carried out for a variety of other purposes, including medical,
pharmacological, chemical, and industrial applications. The methods used include
physical processes such as filtration, sedimentation, and distillation; biological processes such as slow sand filters or biologically active carbon; chemical processes
such as flocculation and chlorination; and the use of electromagnetic
radiation such as ultraviolet light.
Water purification may reduce the concentration of particulate matter including suspended particles, parasites, bacteria, algae, viruses, and fungi as well as reduce the concentration of
a range of dissolved and particulate matter.
https://en.wikipedia.org/wiki/Water_purification
|
Carbonate and bicarbonate alkalinities are decomposed by heat in boiler water
releasing carbon dioxide into the steam. This
gas combines with the condensed steam in process equipment and return lines to
form carbonic acid. This depresses the pH value of the condensate returns and results
in corrosive attack on the equipment and piping.
In general, a dealkalizer is best applied to boilers operating below 700 psi (48 bar). In order to justify installation of a
dealkalizer on low-pressure boilers, the alkalinity content should be above 50 ppm with the amount of make-up
water exceeding 1,000 gallons (approx. 4,000 litres) per day.
Cooling system make-up will also benefit from reduced alkalinity. The
addition of a dealkalizer to a cooling water system will substantially reduce
the amount of acid required to treat the same amount of
water.
https://en.wikipedia.org/wiki/Dealkalization_of_water
Adsorption is present in
many natural, physical, biological and chemical systems and is widely used in
industrial applications such as heterogeneous catalysts,[5][6] activated charcoal,
capturing and using waste heat to
provide cold water for air conditioning and other process requirements (adsorption chillers), synthetic resins,
increasing storage capacity of carbide-derived carbons and water purification.
Adsorption, ion exchange and chromatography are
sorption processes in which certain adsorbates are selectively transferred from
the fluid phase to the surface of insoluble, rigid particles suspended in a
vessel or packed in a column. Pharmaceutical industry applications, which use
adsorption as a means to prolong neurological exposure to specific drugs or
parts thereof,[citation
needed] are
lesser known.
https://en.wikipedia.org/wiki/Adsorption
|
Heterogeneous catalysis is the type of catalysis where the phase of the catalyst differs from the phase of the reactants.[1] This contrasts
with homogeneous catalysis where
the reactants and catalyst exist in the same phase. Phase distinguishes between
not only solid, liquid, and gas components, but also immiscible mixtures (e.g. oil and water), or anywhere an interface is present. Catalysts are
useful because they increase the rate of a reaction[2] without
themselves being consumed and are therefore reusable.
Heterogeneous catalysis typically involves solid phase
catalysts and gas phase reactants.[3] In this case,
there is a cycle of molecular adsorption, reaction, and desorption occurring at
the catalyst surface. Thermodynamics, mass transfer, and heat transfer influence
the rate (kinetics) of reaction.
Heterogeneous catalysis is very important because it
enables faster, large-scale production and the selective product formation.[4] Approximately
35% of the world's GDP is influenced by catalysis.[5] The production
of 90% of chemicals (by volume) is assisted by solid catalysts.[3] The chemical
and energy industries rely heavily on heterogeneous catalysis. For example, the
Haber-Basch process uses metal-based catalysts in the synthesis of ammonia, an important component in fertilizer; 144
million tons of ammonia were produced in 2016.[6]
https://en.wikipedia.org/wiki/Heterogeneous_catalysis
|

|
Leading with Quality, Performance and Cost
Our partners, the
https://www.thermaxglobal.com/ , is a Asia's leading manufacturer & exporter
of TULSION® brand Ion Exchange Resins and a pioneer in the field of MAXTREAT®
brand Fuel & Water treatment chemicals. Thermax also supplies chemicals for
paper industry and for oil field operations. Powered by technological expertise
and capabilities honed over more than 4 decades and backed by a strong dealer
network, the Chemical Division serves customers across the globe and supports
the entire range of Thermax's energy and environment businesses. Backed by
extensive R & D experience and equipped with modern research and
state-of-the-art manufacturing facilities the business has built a client base
in USA, Japan, South East Asia, India & Middle East.

Thermax-Corporate-Brochure-Technology-In-Action.pdf
Chemical Division Product Portfolio :
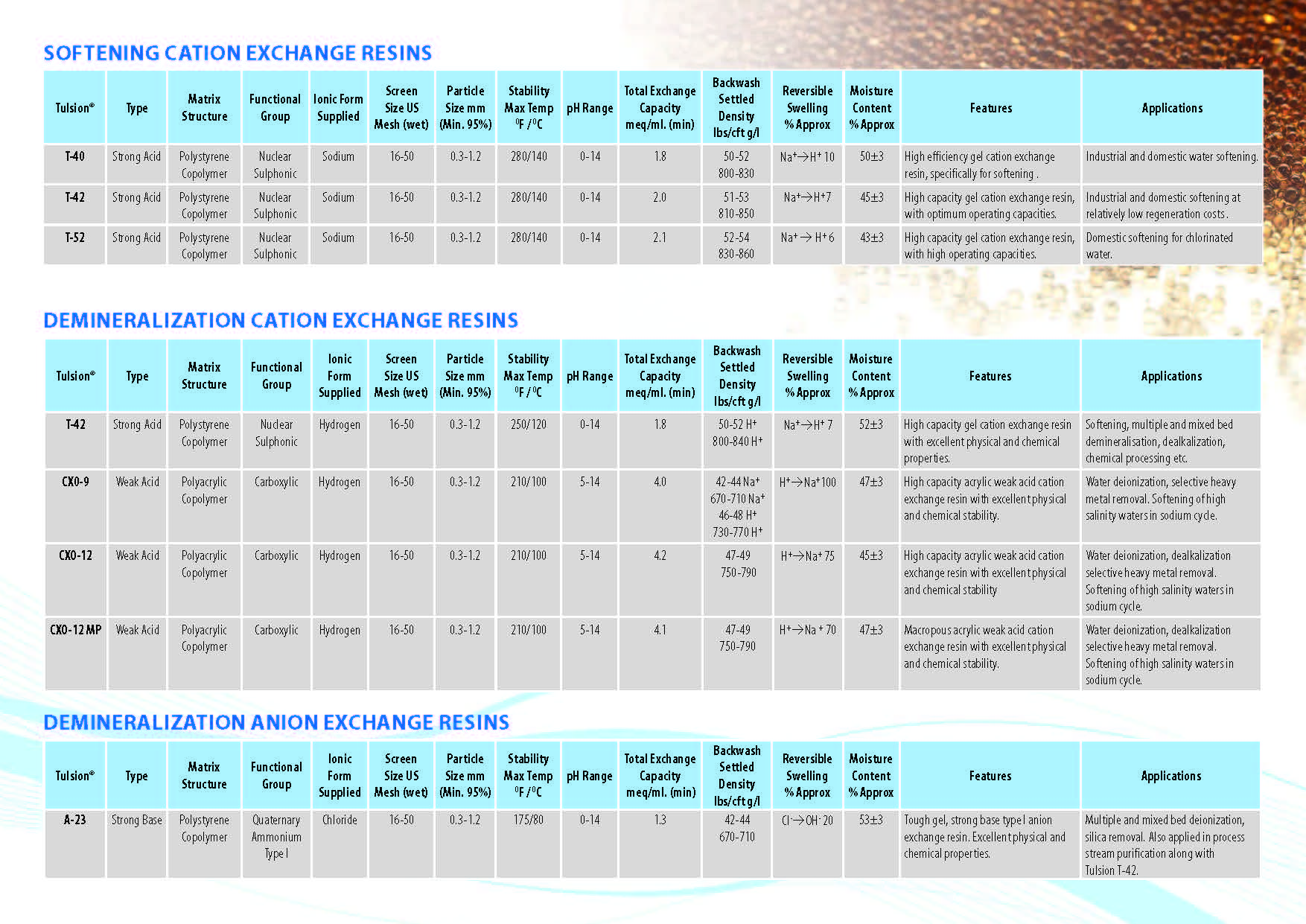
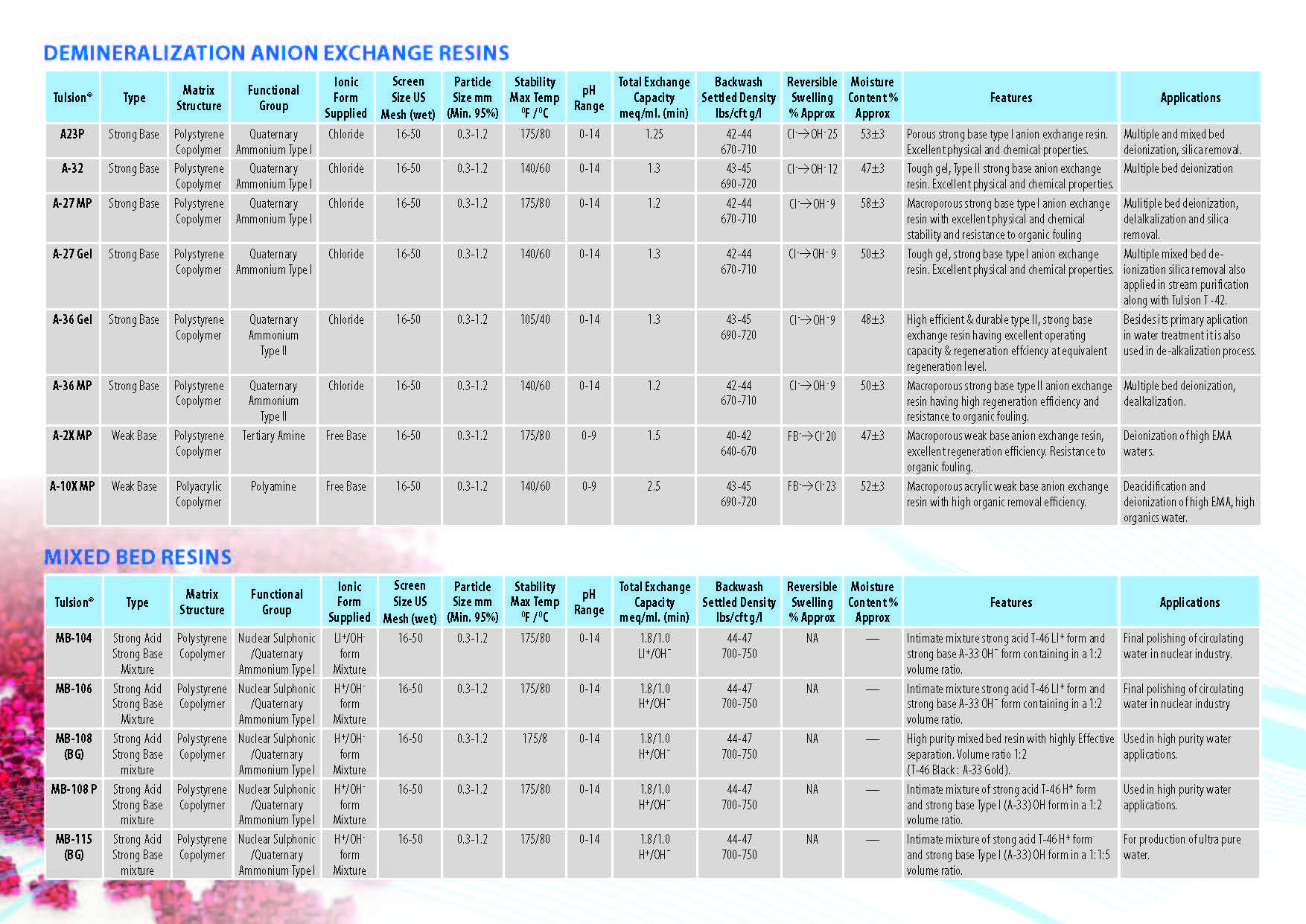
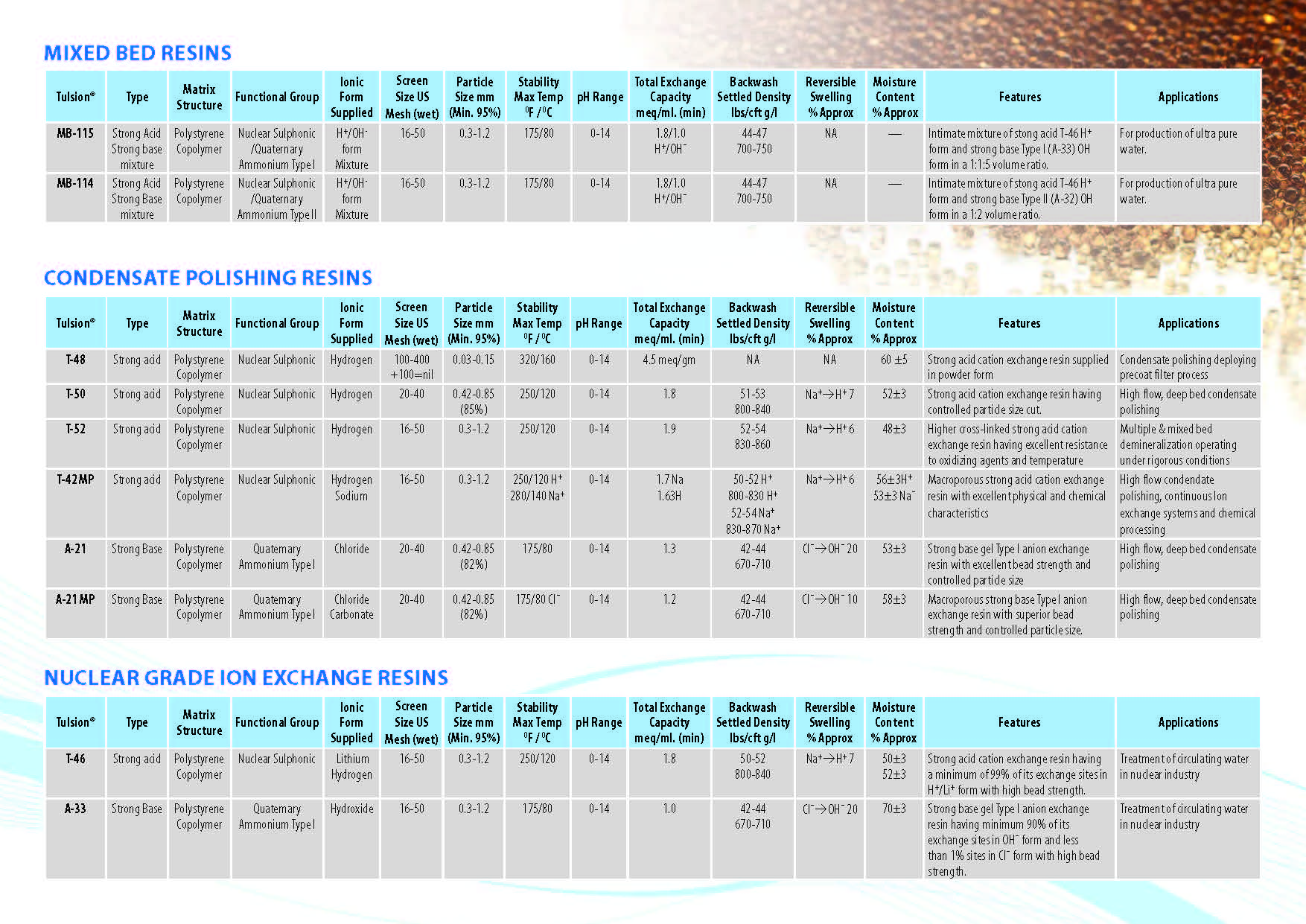
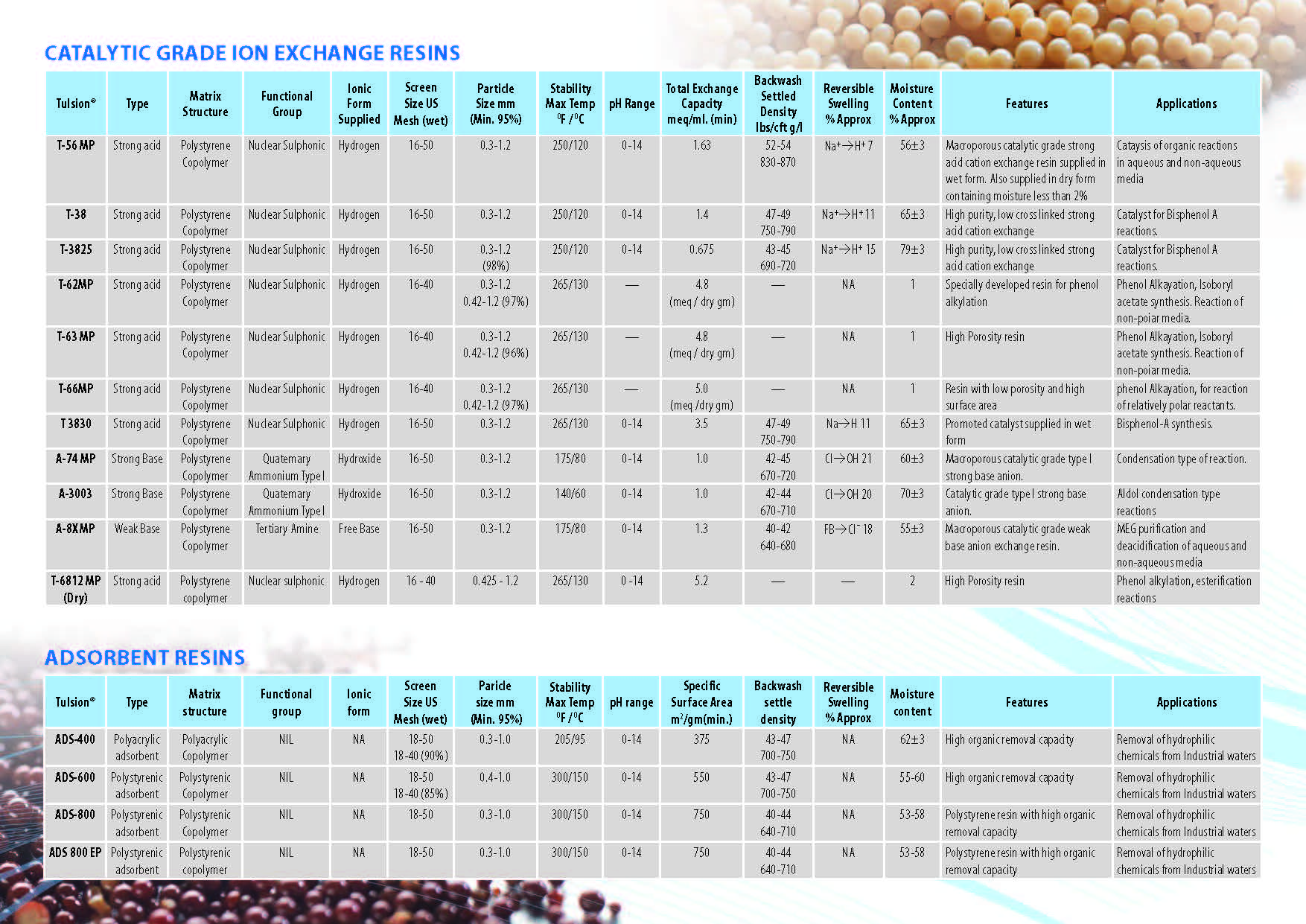
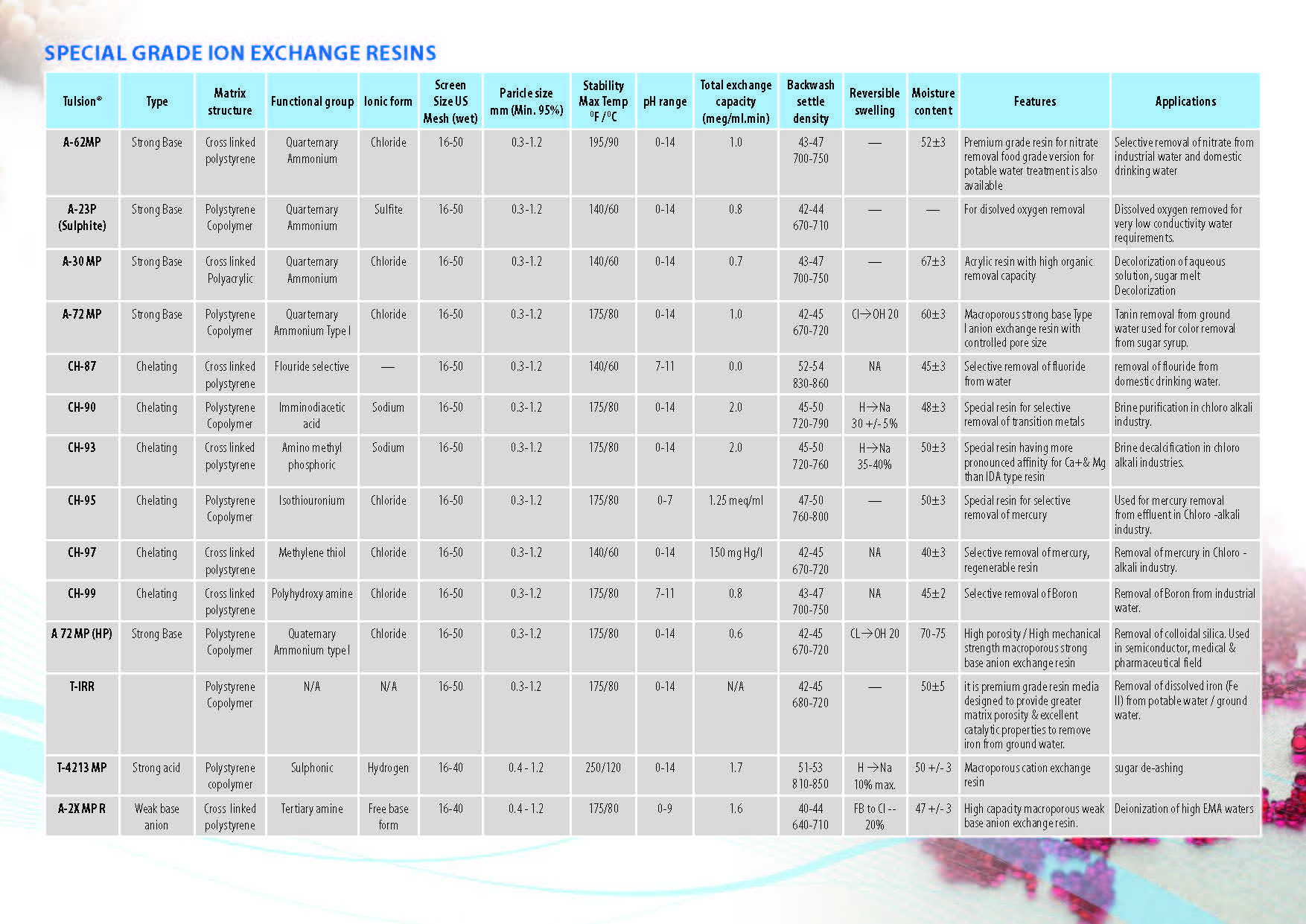
Ion Exchange Resins
:
Ion Exchange Resins Applicationwise
Ion Exchange Resins Typewise
Water Treatment Chemicals
Fuel Additives & Fireside Chemicals
Green Chemicals
Sugar Industry Chemicals
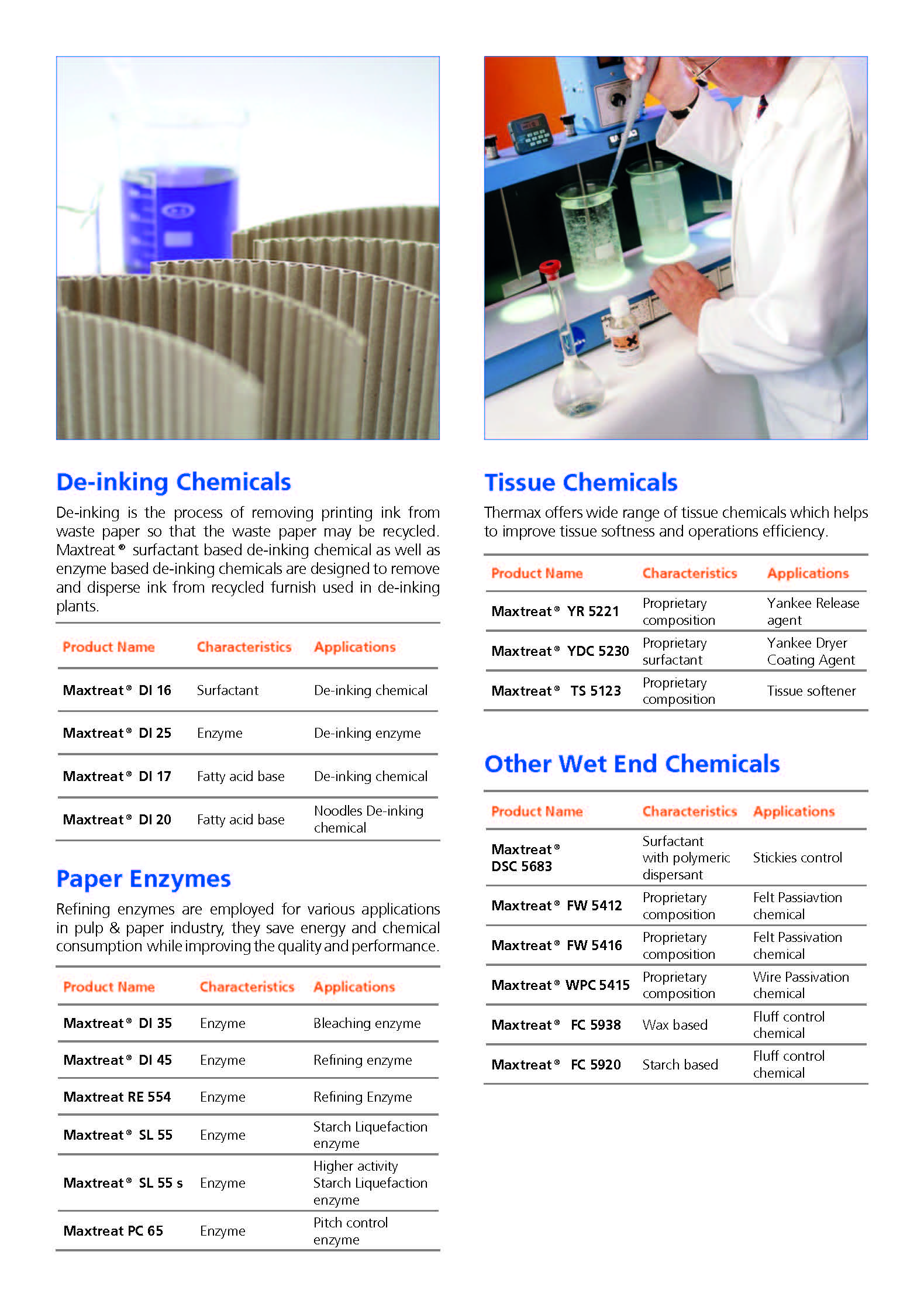
Paper Chemicals
Oil Field Chemicals
Primary Recovery Oil Field Chemicals
Secondary Recovery Oil Field Chemicals
Other Oil Field Chemicals
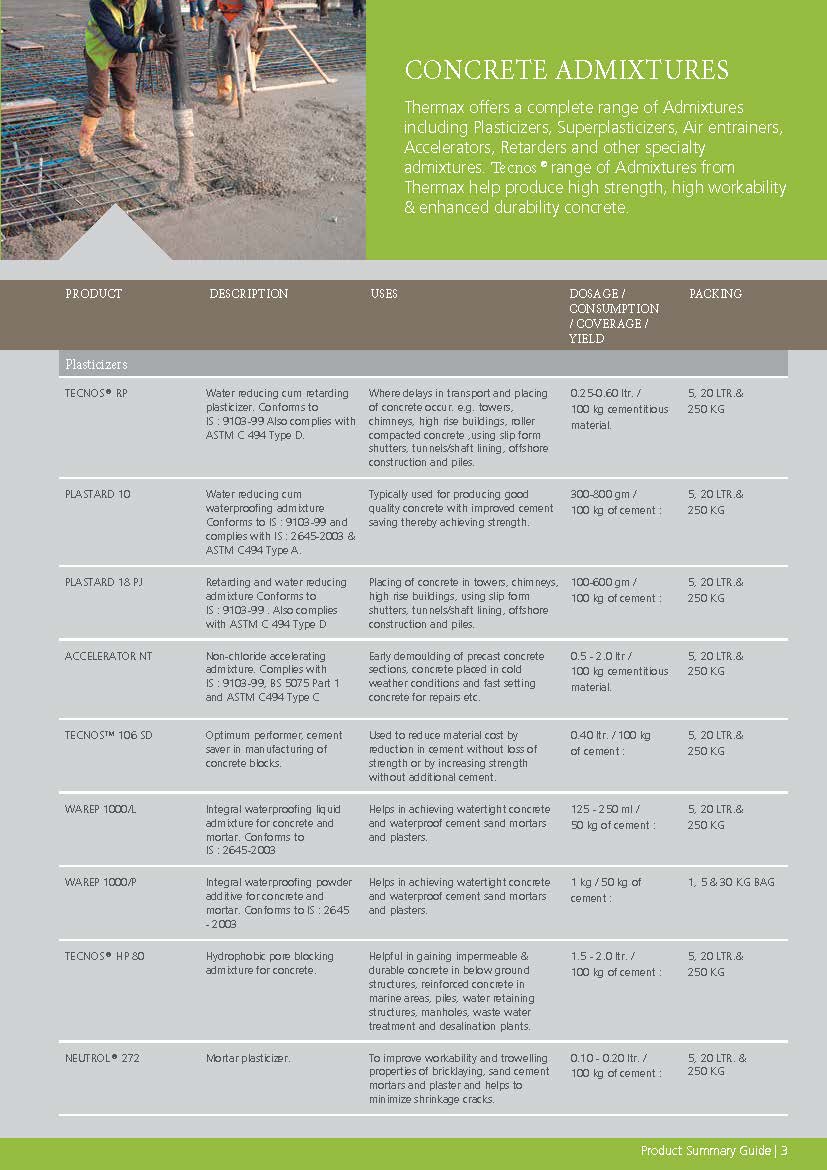
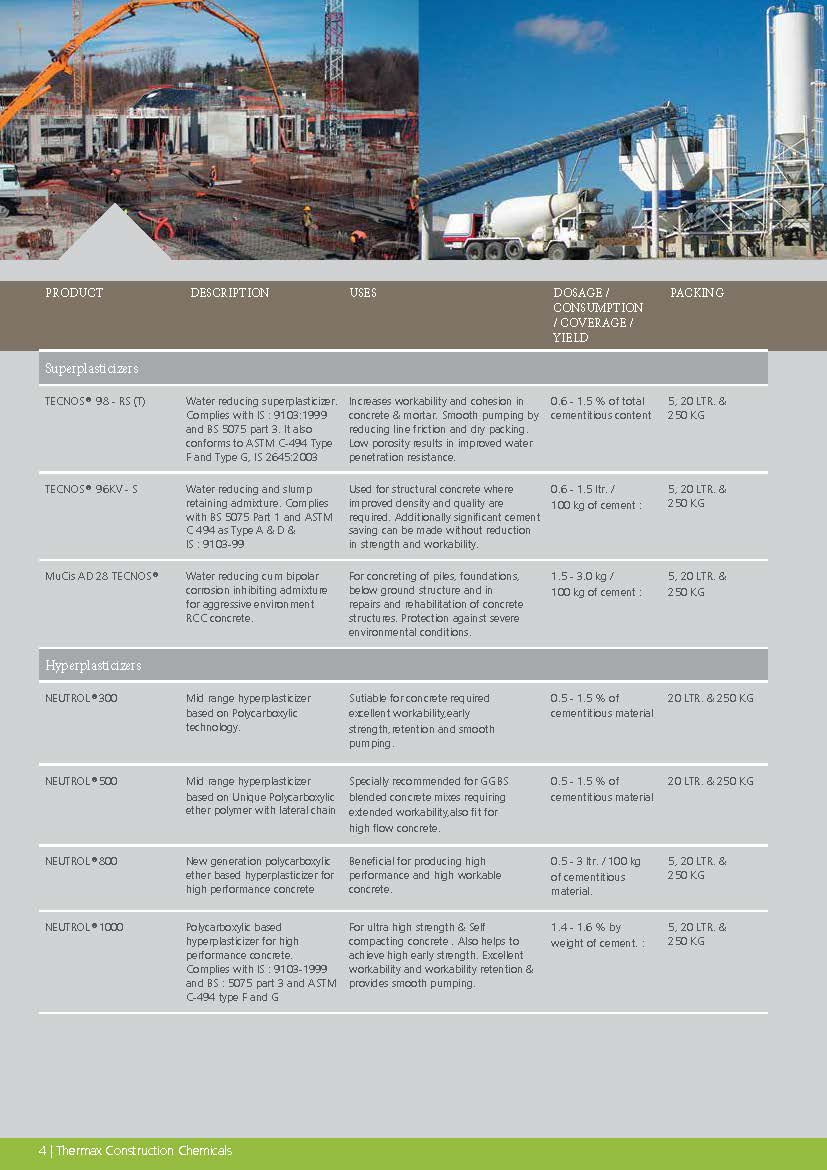
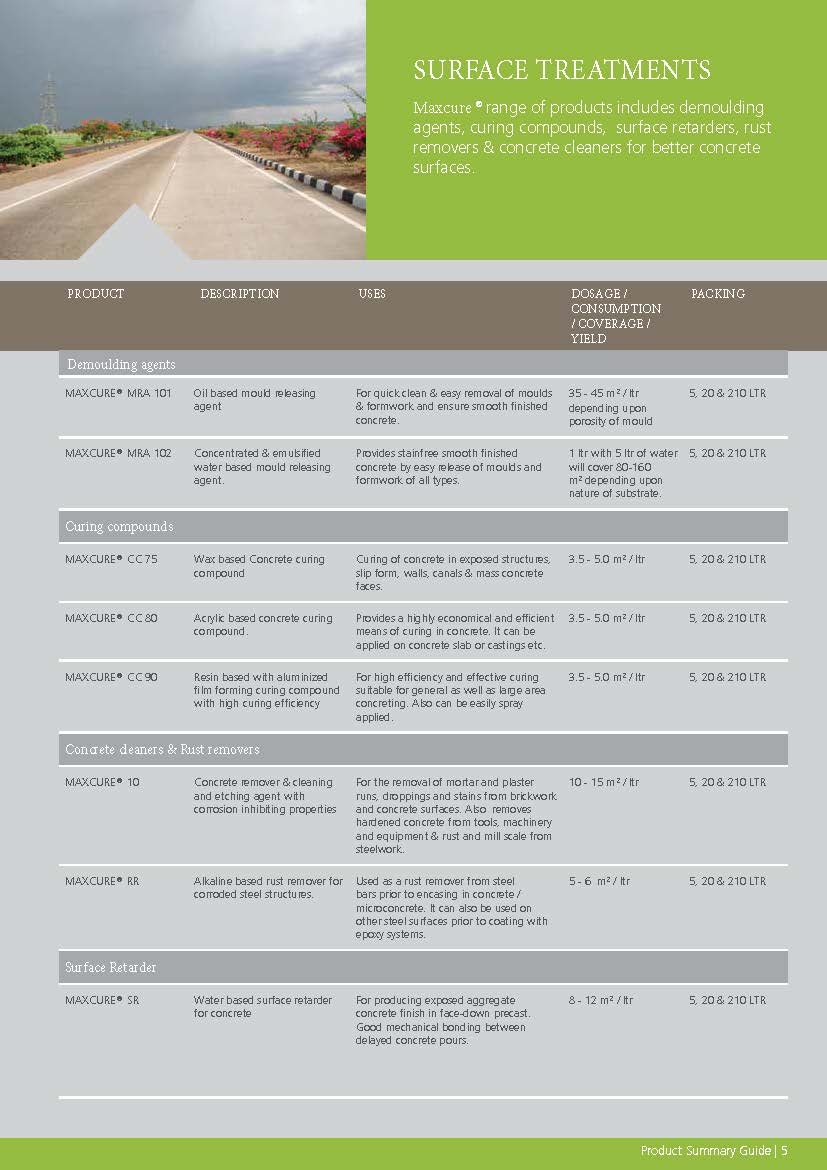
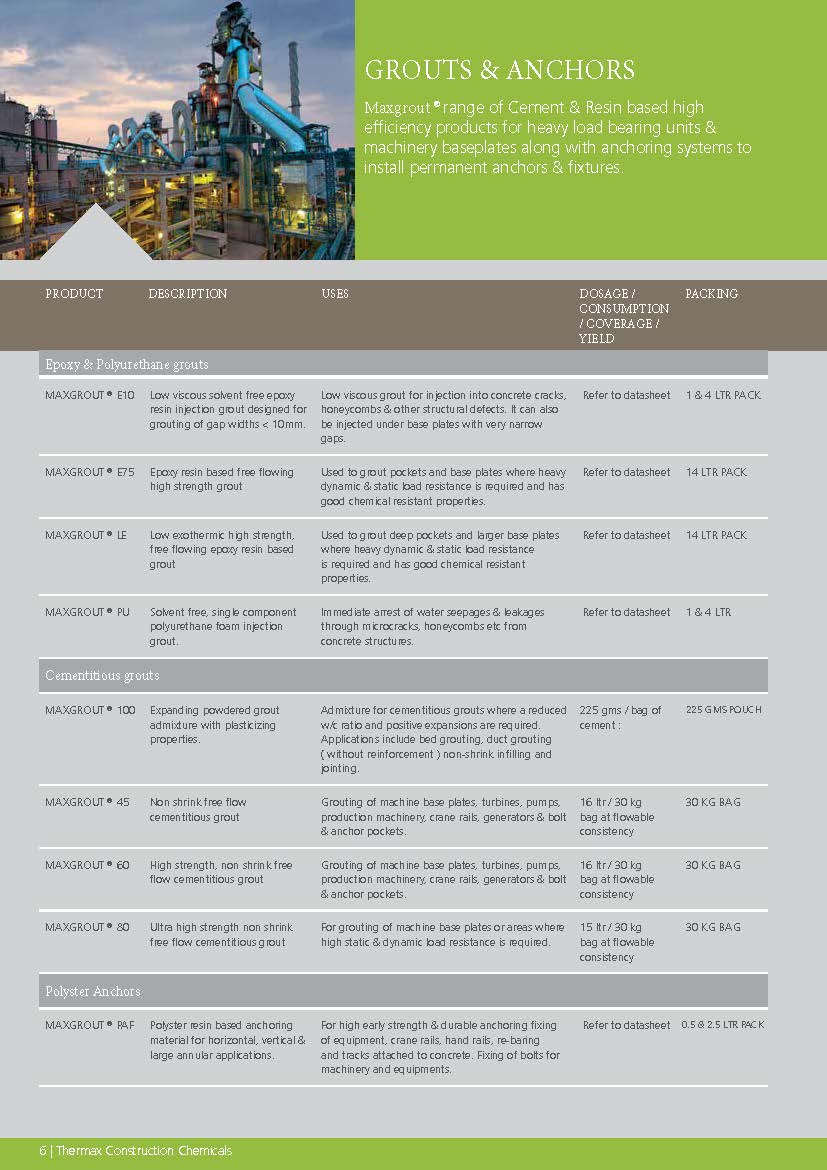
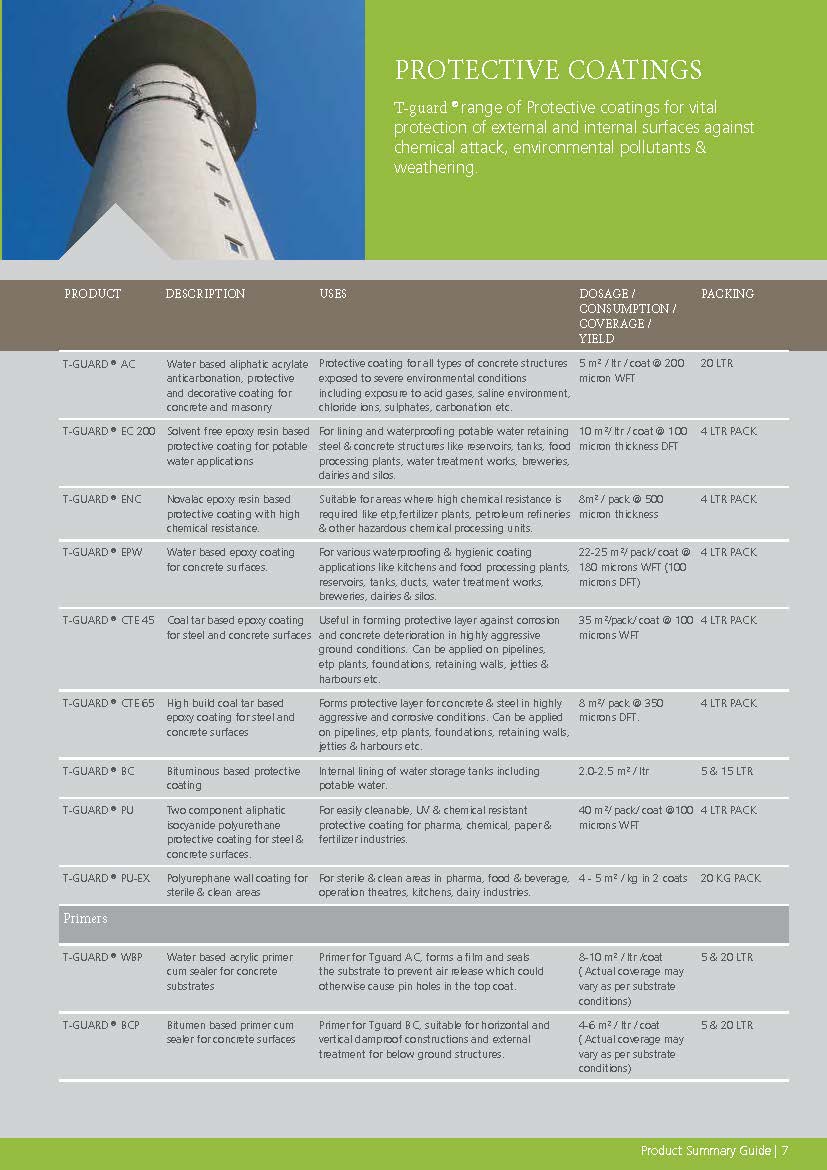
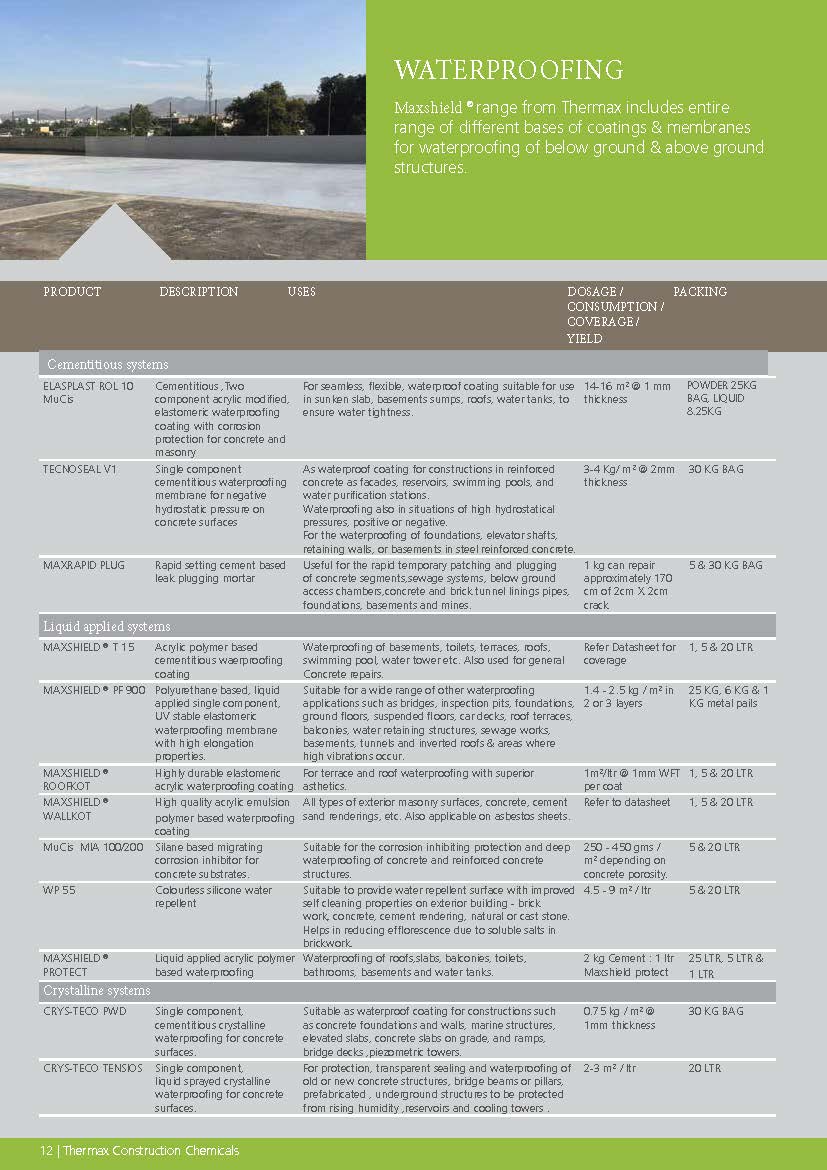
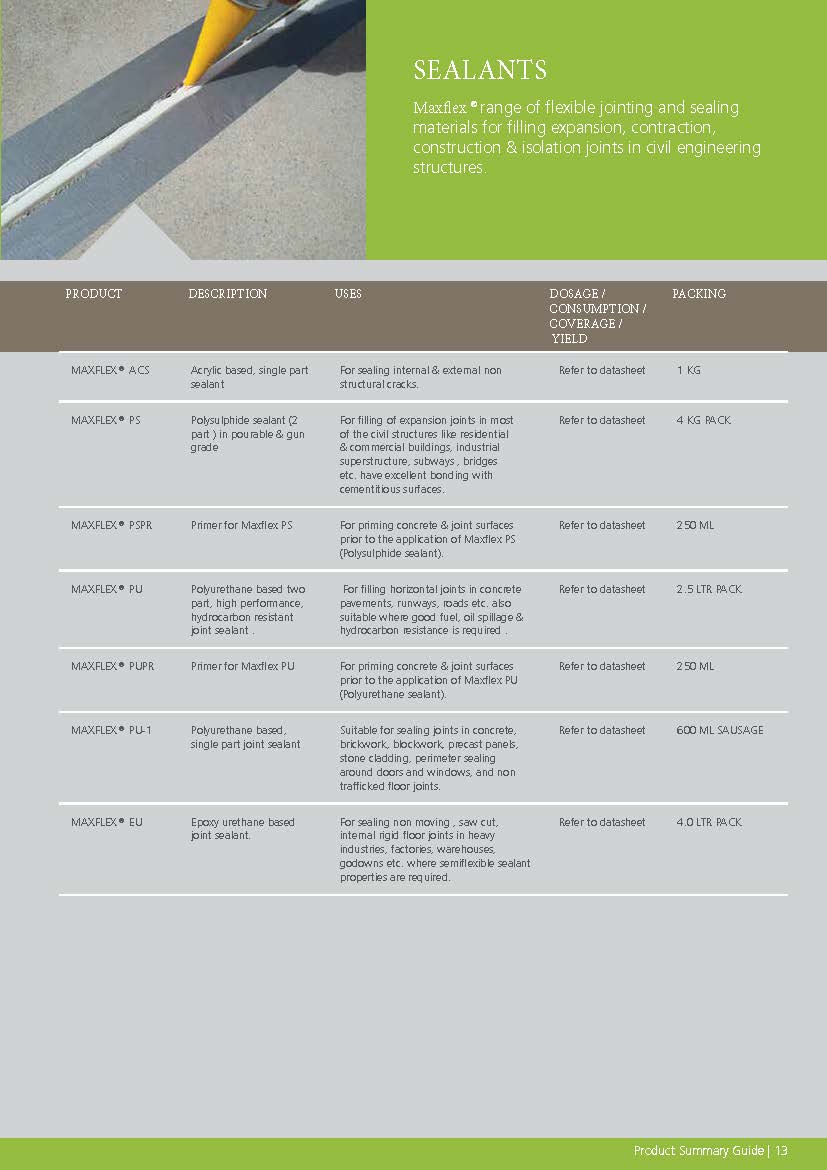
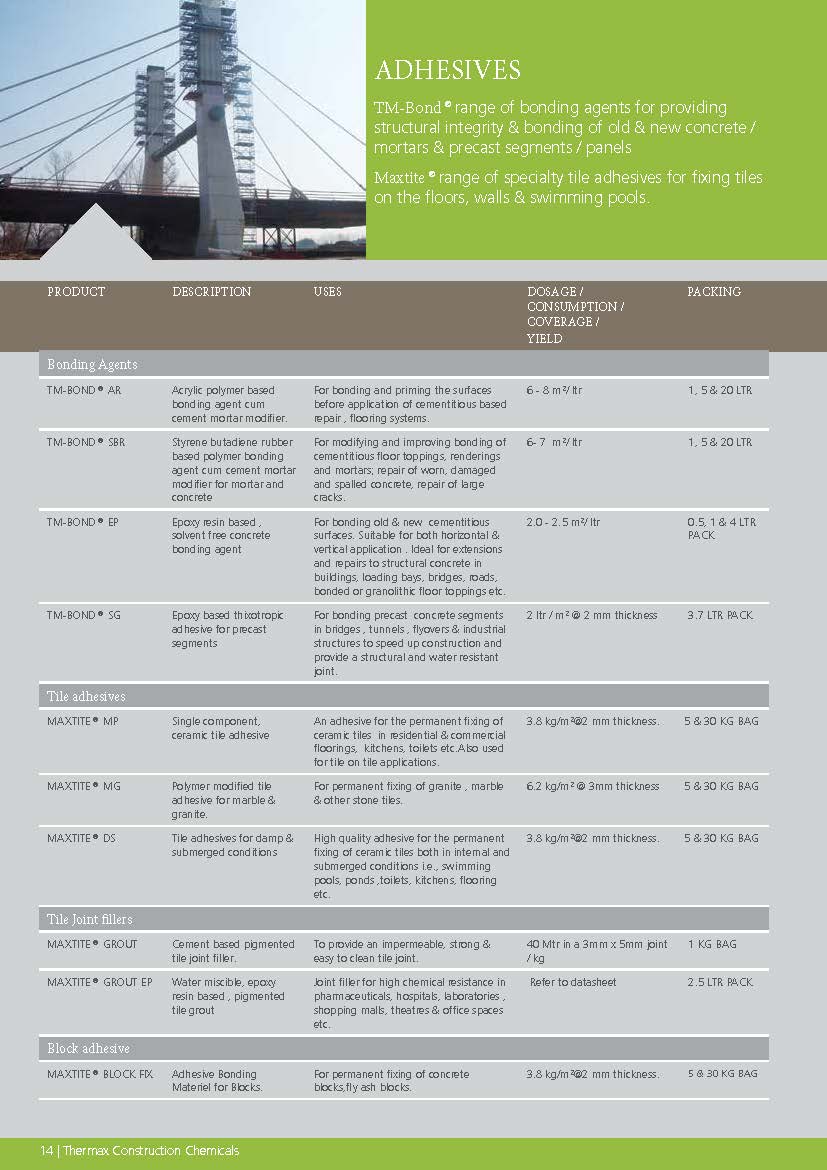
Construction Chemicals
Ion Exchange Resins SDS


Specialty Chemicals for Water and Fuel Management







Chemicals
Paper Industry






Sustainable Construction Solutions



|